Explain How a Pure Substance Is Different From a Mixture
It has a definite composition and constant properties. Pure substances A pure substance has a definite and constant composition like salt or sugar.
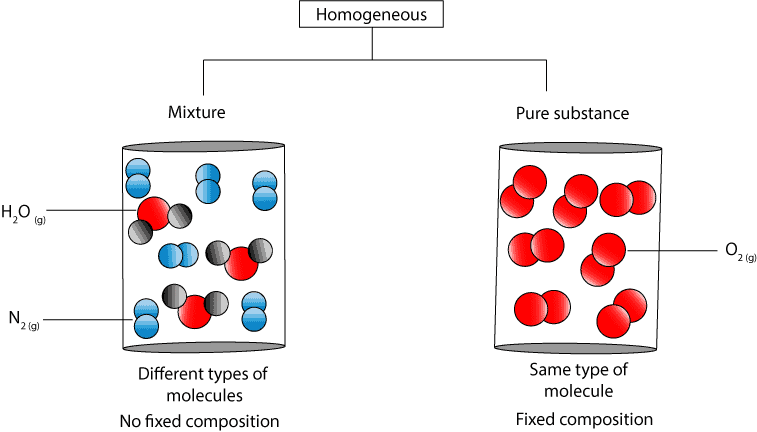
If A Substance Is Homogeneous Is It A Pure Substance
8 rows Difference Between Pure Substance And Mixture.
. The answer is A. It can be the same molecule or atom. Look at the composition of the particles within the substance and determine if the particles are all the same.
All pure substances have characteristic melting and boiling points. For example if you open a container of mixed nuts and pull out a series of small samples and examine them the exact ratio of. However its important to look at them individually in order to understand the nature of these substances better.
You cannot notice that is made up of different substances. A pure substance has constant physical and chemical properties while mixtures have varying physical and chemical properties ie boiling point and melting point. The first called a heterogeneous mixture is distinguished by the fact that different samples of the mixture may have a different composition.
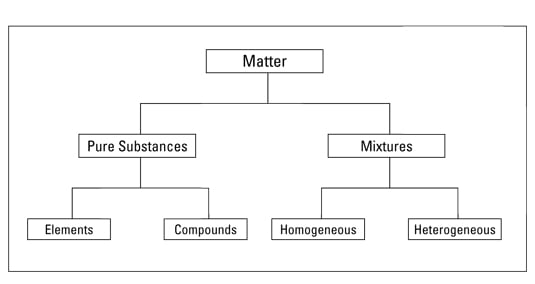
Elements An element is composed of a single kind of atom. An atom is the smallest particle of an element that still has all the properties of the element. In contrast mixtures contain two or more substances so they can be separated.
Another word for a homogeneous mixture is solutionThus a combination of salt and steel wool is a heterogeneous mixture because it is easy to see which particles of the matter are salt. A Mixture contains two or more. A homogeneous mixture looks like it is just one substance.
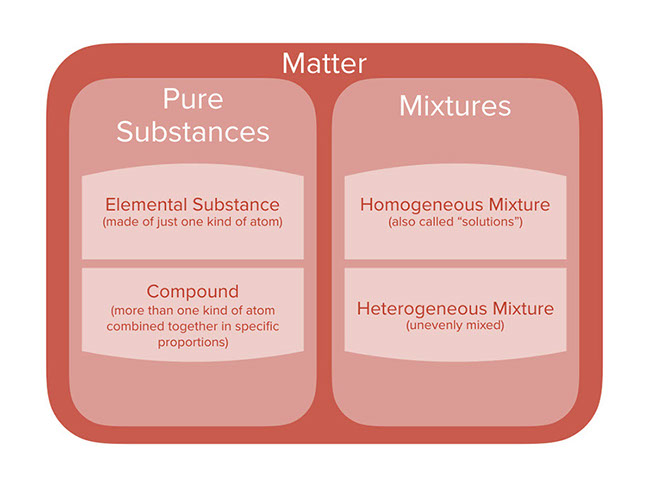
A pure substance consists of only one kind of matter that is all the particles are same. Pure substance can be either an element composed of only one identical type of substances or a compound when two or more different elements chemically combined to produce a new substance with own properties and reactions. Pure substance cannot be separated into two or more substances by any mechanical or physical method.
Mixtures are composed of several kinds of compounds. In a heterogenous mixture it is possible to see the various components. Solid liquid and gas are the three states of.
An example is milk or Gatorade. In contrast substances in a homogeneous mixture can be separated by some methods. Explain the difference between a pure substance and a mixture.
A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves as a single substance. A pure substance is a form of matter that has a definite constant composition and distinctive properties. Pure substances cannot be separated into any other kinds of matter while a mixture is a combination of two or more pure substances.
A pure substance can be either an element or a compound but the composition of a pure substance doesnt vary. Find step-by-step Chemistry solutions and your answer to the following textbook question. You can tell it is made up of more than one substance.
Mixtures fall into two types based on the uniformity of their composition. Pure substance is made up of one component whereas homogeneous mixture is made up of one or more components. The pure substances are the substances that.
In both cases they have only one type of particles. A mixture is composed of more than one substance physically combined. Advertisement Still have questions.
What are the similarities between Pure Substance and Mixture Po. Pure Substance vs Mixture. Pure Substance and Mixture.
Distilled water aluminum foil and table sugar are each made from only one type of particle. Steps to Distinguish between Mixtures and Pure Substances. It cannot be split into simpler substances by physical means.
Homogeneous mixture Solution. However salt water is a mixture because it contains salt as well as water. Pure substances are compounds and elements made up of the same atom or same molecule respectively while mixtures are an assortment of different substances put together.
Pure substances are homogenous. A pure substance contains only one kind of molecule and a mixture is a combination of two or more pure substances. Mixtures can be separated by physical meansA pure substance cannot be separated.
The main difference between pure substance and mixture is that the pure substance consists of one kind of component present in the definite composition whereas the mixture is the substance that consists of more than one component which does not exist in the fixed composition. A pure substance contains only one kind of compound. Find more answers Ask your question Previous Next.
Pure substance cannot be separated into two or more substances by any mechanical or physical method. The main difference between pure substance and mixture lies in their composition. Mixtures show the properties of the pure substances in it.
Therefore the properties are uniform throughout the sample. On the other hand a mixture is a combination of two or more substances in which they retain their properties. 7 rows The matter is divided into two basic categories as pure substance and mixtures.
Mixtures can be separated by physical means is the statement that describes how a pure substance different from a mixture. A pure substance is either a compound or an element. A pure substance is in the purest form and has no impurities in it while mixture has impurities.
1A pure substance is a form of matter that has a fixed chemical composition and a distinct characteristic while a homogeneous mixture is a mixture of two or more compounds with compositions that are uniform or mixed together in such a way that they are indistinguishable from each other. An example is sand at the bottom of a beaker of water. If the particles.
The major difference between pure substances and mixture is that pure substances have a specific composition of constituent while a mixture is the combination of two or more pure substances.
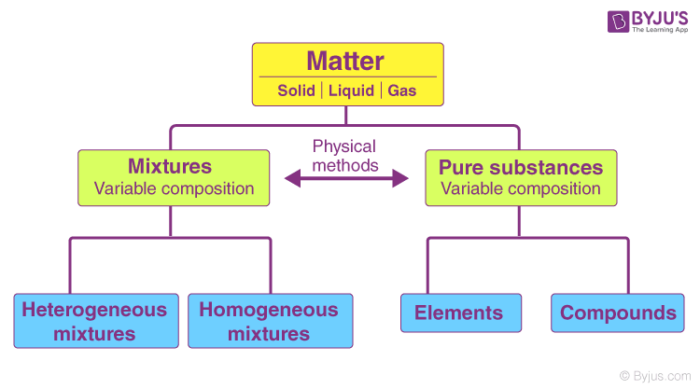
Difference Between Pure Substance And Mixture Definition Composition Properties Examples

What Is Pure Substance Definition Examples Difference Between Pure Substance Mixture
No comments for "Explain How a Pure Substance Is Different From a Mixture"
Post a Comment